DNA damage stress-induced translocation of mutant FUS proteins into cytosolic granules and screening for translocation inhibitors
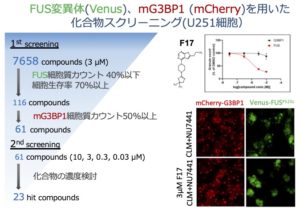
PRKDCがコードするDNA依存性蛋白質リン酸化酵素(DNA-PK)が制御するFUS蛋白質の細胞内局在の動態を検証し、ALS変異を有するFUSの細胞質顆粒への移行を選択的に阻害する化合物スクリーニングを行った結果、23種類の低分子化合物の同定に成功しました。
Fused in sarcoma/translated in liposarcoma (FUS) is an RNA-binding protein, and its mutations are associated with neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), through the DNA damage stress response, aberrant stress granule (SG) formation, etc. We previously reported that translocation of endogenous FUS into SGs was achieved by cotreatment with a DNA double-strand break inducer and an inhibitor of DNA-PK activity. In the present study, we investigated cytoplasmic SG formation using various fluorescent protein-tagged mutant FUS proteins in a human astrocytoma cell (U251) model. While the synergistic enhancement of the migration of fluorescent protein-tagged wild-type FUS to cytoplasmic SGs upon DNA damage induction was observed when DNA-PK activity was suppressed, the fluorescent protein-tagged FUSP525L mutant showed cytoplasmic localization. It migrated to cytoplasmic SGs upon DNA damage induction alone, and DNA-PK inhibition also showed a synergistic effect. Furthermore, analysis of 12 sites of DNA-PK–regulated phosphorylation in the N-terminal LC region of FUS revealed that hyperphosphorylation of FUS mitigated the mislocalization of FUS into cytoplasmic SGs. By using this cell model, we performed screening of a compound library to identify compounds that inhibit the migration of FUS to cytoplasmic SGs but do not affect the localization of the SG marker molecule G3BP1 to cytoplasmic SGs. Finally, we successfully identified 23 compounds that inhibit FUS-containing SG formation without changing normal SG formation.
Authors: Masahiro Nogami, Osamu Sano, Keiko Adachi-Tominari, Yoshika Hayakawa-Yano, Takako Furukawa, Hidehisa Iwata, Kazuhiro Ogi, Hideyuki Okano and Masato Yano
Journal: Frontiers in Molecular Neuroscience (2022)
DOI: https://doi.org/10.3389/fnmol.2022.953365
投稿者プロフィール

- 新潟大学 医学部 准教授
最新の投稿
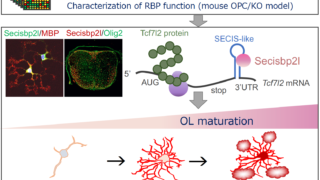 研究成果2023.11.26Sbp2l contributes to oligodendrocyte maturation through translational control in Tcf7l2 signaling
研究成果2023.11.26Sbp2l contributes to oligodendrocyte maturation through translational control in Tcf7l2 signaling ノンドメインブログ2023.09.08古(いにしえ)の論文より
ノンドメインブログ2023.09.08古(いにしえ)の論文より 令和4年度 (FY2022)2022.12.21DNA damage stress-induced translocation of mutant FUS proteins into cytosolic granules and screening for translocation inhibitors
令和4年度 (FY2022)2022.12.21DNA damage stress-induced translocation of mutant FUS proteins into cytosolic granules and screening for translocation inhibitors ノンドメインブログ2022.07.10非ドメイン生物学における美の追求
ノンドメインブログ2022.07.10非ドメイン生物学における美の追求

