Phase 1/2a clinical trial in ALS with ropinirole, a drug candidate identified by iPSC drug discovery
GPCR(IDP)の一種であるdopamine D2 receptorに対するagonist(ropinirole hydrochloride)が、筋萎縮性側索硬化症(ALS)へ与える影響について、基礎-臨床一体型の研究により明らかにしました。
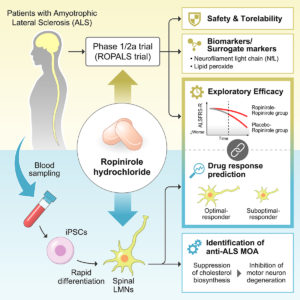
Abstract: iPSC-based drug discovery led to a phase 1/2a trial of ropinirole in ALS. 20 participants with sporadic ALS received ropinirole or placebo for 24 weeks in the double-blind period to evaluate safety, tolerability, and therapeutic effects. Adverse events were similar in both groups. During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R, which assesses the functional status of ALS patients, was not different from that in the placebo group. However, in the open-label extension period, the ropinirole group showed significant suppression of ALSFRS-R decline and an additional 27.9 weeks of disease-progression-free survival. iPSC-derived motor neurons from participants showed dopamine D2 receptor expression and a potential involvement of the SREBP2-cholesterol pathway in therapeutic effects. Lipid peroxide represents a clinical surrogate marker to assess disease progression and drug efficacy. Limitations include small sample sizes and high attrition rates in the open-label extension period, requiring further validation.
Author: Morimoto S, Takahashi S, Ito D, Daté Y, Okada K, Kato C, Nakamura S, Ozawa F, Chyi CM, Nishiyama A, Suzuki N, Fujimori K, Kondo T, Takao M, Hirai M, Kabe Y, Suematsu M, Jinzaki M, Aoki M, Fujiki Y, Sato Y, Suzuki N, Nakahara J; Pooled Resource Open-Access ALS Clinical Trials Consortium; Okano H.
Journal: Cell Stem Cell 2023;30(6):766-780.
投稿者プロフィール

- Project Associate Professor
-
Satoru Morimoto, M.D., Ph.D.
Keio University Regenerative Medicine Research Center (KRM)
Project associate professorResearch Gate Building TONOMACHI 2, 4B, 3-25-10, Tonomachi, Kawasaki-ku, Kawasaki-shi, Kanagawa,
210-0821, Japan
最新の投稿
 ノンドメインブログ2026.02.16第八節 あらゆる可能性に向けた 再生医療へのどとう怒濤の挑戦
ノンドメインブログ2026.02.16第八節 あらゆる可能性に向けた 再生医療へのどとう怒濤の挑戦 令和7年度(FY2025)2026.01.29Diagnostic potential of cryptic exon-derived peptides in serum extracellular vesicles for sporadic amyotrophic lateral sclerosis
令和7年度(FY2025)2026.01.29Diagnostic potential of cryptic exon-derived peptides in serum extracellular vesicles for sporadic amyotrophic lateral sclerosis ノンドメインブログ2026.01.01第七節 臨床に応用できて価値がある 再生医療への本格的挑戦
ノンドメインブログ2026.01.01第七節 臨床に応用できて価値がある 再生医療への本格的挑戦 令和7年度(FY2025)2025.12.05A genome-wide association study identifies the GPM6A locus associated with age at onset in ALS
令和7年度(FY2025)2025.12.05A genome-wide association study identifies the GPM6A locus associated with age at onset in ALS