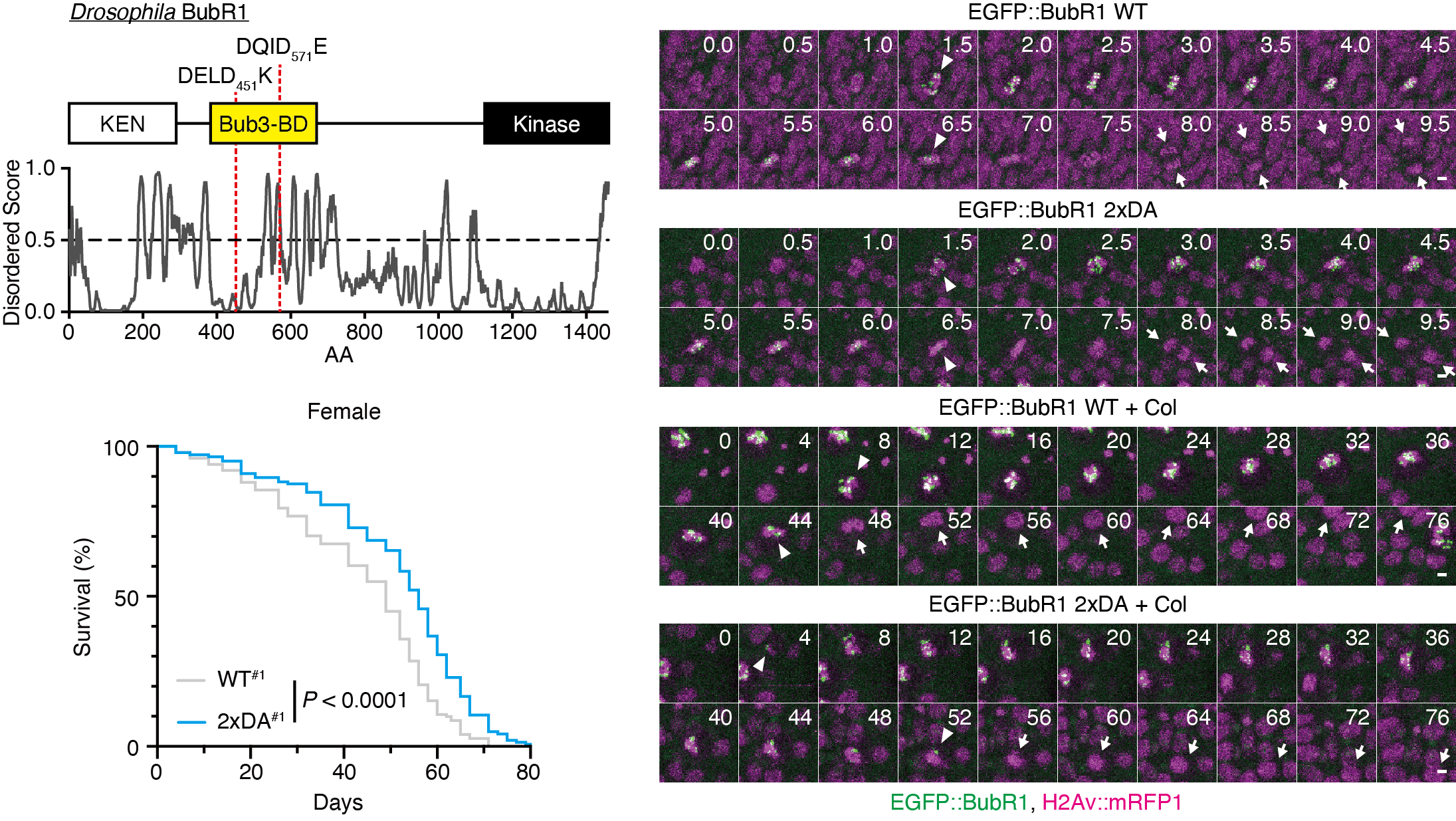
Caspase cleaves Drosophila BubR1 to modulate spindle assembly checkpoint function and lifespan of the organism
紡錘体チェックポイント構成因子BubR1をショウジョウバエにおける新規カスパーゼ基質として同定しました. また, 非切断変異体系統の作出から, 切断によって紡錘体チェックポイント機能と個体寿命が制限されることを見出しました. さらに, 近接依存性標識法TurboIDを用い, BubR1を嗜好生高く切断する実行カスパーゼが, BubR1に近接することを生体で示しました.
Abstract: Caspases cleave over 1500 substrates in the human proteome in both lethal and non-lethal scenarios. However, reports of the physiological consequences of substrate cleavage are limited. Additionally, the manner in which caspase cleaves only a subset of substrates in the non-lethal scenario remains to be elucidated. BubR1, a spindle assembly checkpoint component, is a caspase substrate in humans, the physiological function of which remains unclear. Here, we found that caspases, especially Drice, cleave Drosophila BubR1 between the N-terminal KEN box motif and C-terminal kinase domain. By using proximity labelling, we found that Drice, but not Dcp-1, is in proximity to BubR1, suggesting that protein proximity facilitates substrate preference. The cleaved fragments displayed altered subcellular localization and protein–protein interactions. Flies that harboured cleavage-resistant BubR1 showed longer duration of BubR1 localization to the kinetochore upon colchicine treatment. Furthermore, these flies showed extended lifespan. Thus, we propose that the caspase-mediated cleavage of BubR1 limits spindle assembly checkpoint and organismal lifespan. Our results highlight the importance of the individual analysis of substrates in vivo to determine the biological significance of caspase-dependent non-lethal cellular processes.
Authors: Natsuki Shinoda, Misuzu Horikoshi, Yusuke Taira, Masaya Muramoto, Shoshiro Hirayama, Shigeo Murata and Masayuki Miura
投稿者プロフィール

最新の投稿
 令和7年度(FY2025)2025.06.19Executioner caspase is proximal to Fasciclin 3 which facilitates non-lethal activation in Drosophila olfactory receptor neurons
令和7年度(FY2025)2025.06.19Executioner caspase is proximal to Fasciclin 3 which facilitates non-lethal activation in Drosophila olfactory receptor neurons ノンドメインブログ2023.09.06細胞自動セグメンテーションAI
ノンドメインブログ2023.09.06細胞自動セグメンテーションAI 令和5年度 (FY2023)2023.05.16Caspase cleaves Drosophila BubR1 to modulate spindle assembly checkpoint function and lifespan of the organism
令和5年度 (FY2023)2023.05.16Caspase cleaves Drosophila BubR1 to modulate spindle assembly checkpoint function and lifespan of the organism ノンドメインブログ2022.07.03危険なおもちゃ(カスパーゼ)の扱い方
ノンドメインブログ2022.07.03危険なおもちゃ(カスパーゼ)の扱い方