NMR characterization of the structure of the intrinsically disordered region of human origin recognition complex subunit 1, hORC1, and of its interaction with G-quadruplex DNAs

Human origin recognition complex (hORC) binds to the DNA replication origin and then initiates DNA replication. However, hORC does not exhibit DNA sequence-specificity and how hORC recognizes the replication origin on genomic DNA remains elusive. Previously, we found that hORC recognizes G-quadruplex structures potentially formed near the replication origin. Then, we showed that hORC subunit 1 (hORC1) preferentially binds to G-quadruplex DNAs using a hORC1 construct comprising residues 413 to 511 (hORC1413−511). Here, we investigate the structural characteristics of hORC1413−511 in its free and complex forms with G-quadruplex DNAs. Circular dichroism and nuclear magnetic resonance (NMR) spectroscopic studies indicated that hORC1413−511 is disordered except for a short α-helical region in both the free and complex forms. NMR chemical shift perturbation (CSP) analysis suggested that basic residues, arginines and lysines, and polar residues, serines and threonines, are involved in the G-quadruplex DNA binding. Then, this was confirmed by mutation analysis. Interestingly, CSP analysis indicated that hORC1413−511 binds to both parallel- and (3 + 1)-type G-quadruplex DNAs using the same residues, and thereby in the same manner. Our study suggests that hORC1 uses its intrinsically disordered G-quadruplex binding region to recognize parallel-type and (3 + 1)-type G-quadruplex structures at replication origin.
Authors: Afaf Eladl, Yudai Yamaoki, Keisuke Kamba, Shoko Hoshino, Haruka Horinouchi, Keiko Kondo, Show Wada, Takashi Nagata, matato Katahira
Journal: j.bbrc. 2023.10.044
投稿者プロフィール

最新の投稿
 研究成果2023.10.20NMR characterization of the structure of the intrinsically disordered region of human origin recognition complex subunit 1, hORC1, and of its interaction with G-quadruplex DNAs
研究成果2023.10.20NMR characterization of the structure of the intrinsically disordered region of human origin recognition complex subunit 1, hORC1, and of its interaction with G-quadruplex DNAs 研究成果2023.09.29Shedding light on the base-pair opening dynamics of nucleic acids in living human cells
研究成果2023.09.29Shedding light on the base-pair opening dynamics of nucleic acids in living human cells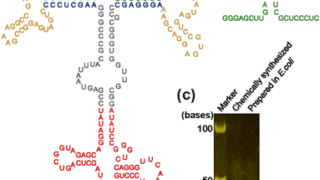 研究成果2023.09.29Detection of interaction between an RNA aptamer and its target compound in living human cells using 2D in-cell NMR
研究成果2023.09.29Detection of interaction between an RNA aptamer and its target compound in living human cells using 2D in-cell NMR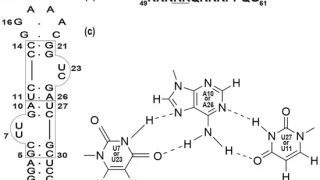 研究成果2023.09.29Complex Formation of an RNA Aptamer with a Part of HIV-1 Tat through Induction of Base Triples in Living Human Cells Proven by In-Cell NMR
研究成果2023.09.29Complex Formation of an RNA Aptamer with a Part of HIV-1 Tat through Induction of Base Triples in Living Human Cells Proven by In-Cell NMR