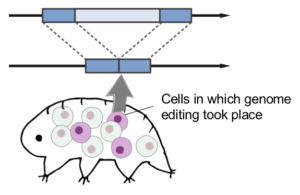
Stress-dependent cell stiffening by tardigrade tolerance proteins that reversibly form a filamentous network and gel

クマムシの乾燥耐性に関わるタンパク質として、脱水様ストレスに応答して可逆的に集合するタンパク質群を分離する手法を樹立し、多くの「非ドメイン型タンパク質」群を同定することに成功しました。そのなかに含まれていたカーズ(CAHS)タンパク質の一部はゆるやかな脱水をもたらす高浸透圧ストレスに応答して細胞内で可逆的に繊維を形成し、細胞の機械的な強度および高浸透圧ストレス耐性を向上させることを明らかにしました。環境ストレスに応答してダイナミックに高次構造体を形成することで、オンデマンドに細胞に機械的な安定性を付与するという「非ドメイン型タンパク質」による新たな耐性メカニズムを提唱しました。
Tardigrades are able to tolerate almost complete dehydration by entering a reversible ametabolic state called anhydrobiosis and resume their animation upon rehydration. Dehydrated tardigrades are exceptionally stable and withstand various physical extremes. Although trehalose and late embryogenesis abundant (LEA) proteins have been extensively studied as potent protectants against dehydration in other anhydrobiotic organisms, tardigrades produce high amounts of tardigrade-unique protective proteins. Cytoplasmic-abundant heat-soluble (CAHS) proteins are uniquely invented in the lineage of eutardigrades, a major class of the phylum Tardigrada and are essential for their anhydrobiotic survival. However, the precise mechanisms of their action in this protective role are not fully understood. In the present study, we first postulated the presence of tolerance proteins that form protective condensates via phase separation in a stress-dependent manner and searched for tardigrade proteins that reversibly form condensates upon dehydration-like stress. Through a comprehensive search using a desolvating agent, trifluoroethanol (TFE), we identified 336 proteins, collectively dubbed “TFE-Dependent ReversiblY condensing Proteins (T-DRYPs).” Unexpectedly, we rediscovered CAHS proteins as highly enriched in T-DRYPs, 3 of which were major components of T-DRYPs. We revealed that these CAHS proteins reversibly polymerize into many cytoskeleton-like filaments depending on hyperosmotic stress in cultured cells and undergo reversible gel-transition in vitro. Furthermore, CAHS proteins increased cell stiffness in a hyperosmotic stress-dependent manner and counteract the cell shrinkage caused by osmotic pressure, and even improved the survival against hyperosmotic stress. The conserved putative helical C-terminal region is necessary and sufficient for filament formation by CAHS proteins, and mutations disrupting the secondary structure of this region impaired both the filament formation and the gel transition. On the basis of these results, we propose that CAHS proteins are novel cytoskeleton-like proteins that form filamentous networks and undergo gel-transition in a stress-dependent manner to provide on-demand physical stabilization of cell integrity against deformative forces during dehydration and could contribute to the exceptional physical stability in a dehydrated state.
Authors: Akihiro Tanaka,Tomomi Nakano,Kento Watanabe,Kazutoshi Masuda,Gen Honda,Shuichi Kamata,Reitaro Yasui,Hiroko Kozuka-Hata,Chiho Watanabe,Takumi Chinen,Daiju Kitagawa,Satoshi Sawai,Masaaki Oyama,Miho Yanagisawa,Takekazu Kunieda
Journal: PLOS Biology (2022) 20(9): e3001780
DOI: https://doi.org/10.1371/journal.pbio.3001780
日本語プレスリリース「クマムシ耐性タンパク質によるストレスに応答した細胞の硬化」
英文プレスリリース “How tardigrades bear dehydration.”
投稿者プロフィール

最新の投稿
 令和6年度(FY2024)2024.06.25Single-step generation of homozygous knockout/knock-in individuals in an extremotolerant parthenogenetic tardigrade using DIPA-CRISPR
令和6年度(FY2024)2024.06.25Single-step generation of homozygous knockout/knock-in individuals in an extremotolerant parthenogenetic tardigrade using DIPA-CRISPR ノンドメインブログ2024.05.25虚心坦懐
ノンドメインブログ2024.05.25虚心坦懐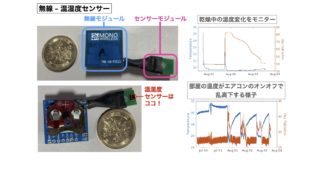 ノンドメインブログ2023.08.30無線で温湿度情報を飛ばしてクラウドへ
ノンドメインブログ2023.08.30無線で温湿度情報を飛ばしてクラウドへ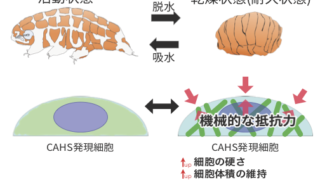 令和4年度 (FY2022)2022.09.22Stress-dependent cell stiffening by tardigrade tolerance proteins that reversibly form a filamentous network and gel
令和4年度 (FY2022)2022.09.22Stress-dependent cell stiffening by tardigrade tolerance proteins that reversibly form a filamentous network and gel