Executioner caspase is proximal to Fasciclin 3 which facilitates non-lethal activation in Drosophila olfactory receptor neurons
細胞死実行因子であるカスパーゼは細胞死以外にも多様な細胞機能を制御します. しかし, その「非」細胞死性の活性化を可能とする分子機構は未だ明らかではありませんでした. 本研究では, ショウジョウバエを用いて, カスパーゼと近接するタンパク質を近接依存性標識法TurboIDにより網羅的に同定しました. 同定した因子とカスパーゼとの遺伝生化学的な解析から, カスパーゼの活性化を細胞内の一部に区画化することが, 「非」細胞死性の活性化を可能とする分子機構である可能性を示しました.
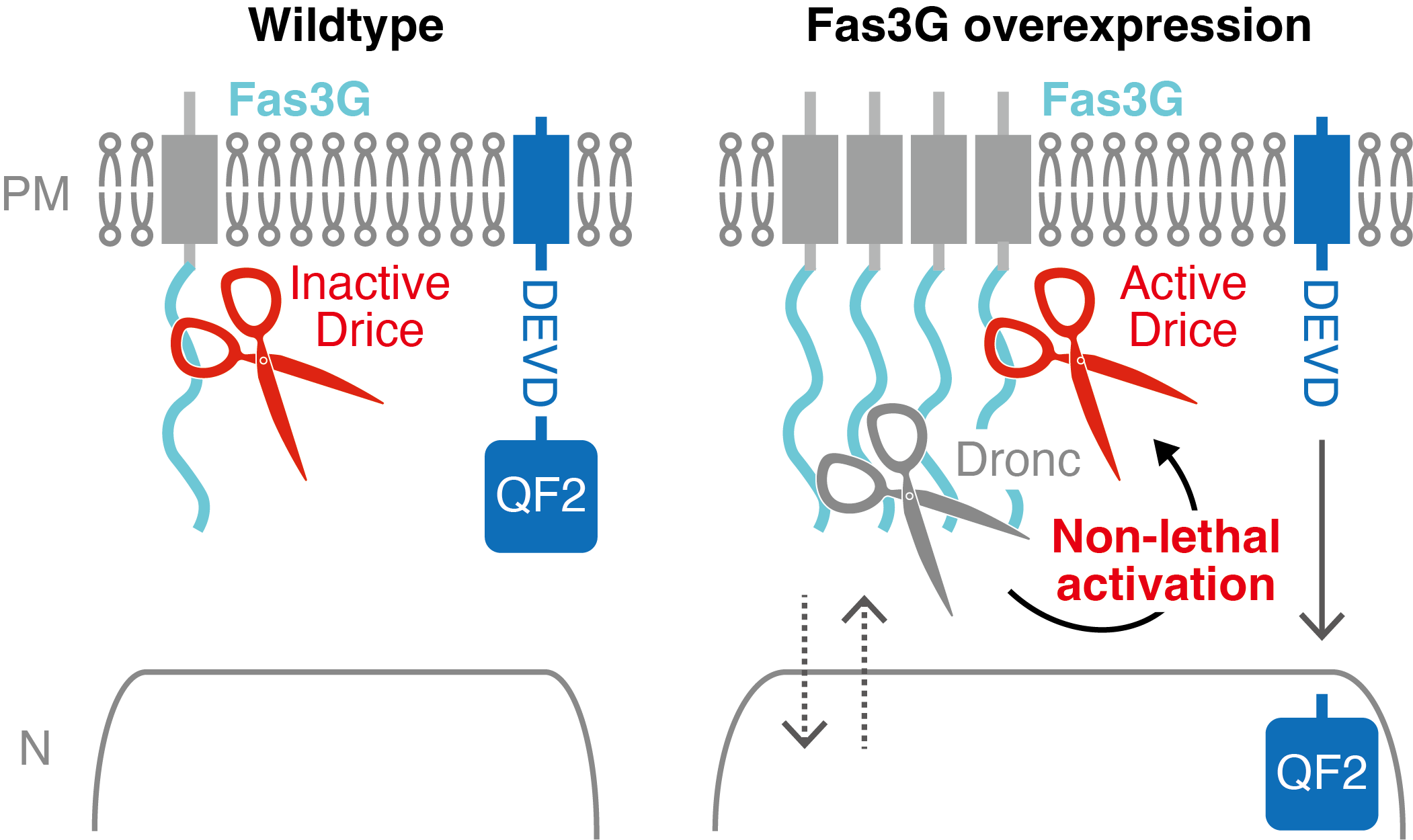
Abstract: The nervous system undergoes functional modification independent of cell turnover. Caspase participates in reversible neuronal modulation via non-lethal activation. However, the mechanism that enables non-lethal activation remains unclear. Here, we analyzed proximal proteins of Drosophila executioner caspase in the adult brain using TurboID. We discovered that executioner caspase Drice is, as an inactive proform, proximal to cell membrane proteins, including a specific splicing isoform of cell adhesion molecule Fasciclin 3 (Fas3), Fas3G. To investigate whether sequestration of executioner caspase to plasma membrane of axons is the mechanism for non-lethal activation, we developed a Gal4-Manipulated Area-Specific CaspaseTracker/CasExpress system for sensitive monitoring of caspase activity near the plasma membrane. We demonstrated that Fas3G overexpression promotes caspase activation in olfactory receptor neurons without killing them, by inducing expression of initiator caspase Dronc, which also comes close to Fas3G. Physiologically, Fas3G overexpression-facilitated non-lethal caspase activation suppresses innate olfactory attraction behavior. Our findings suggest that subcellularly restricted caspase activation, defined by caspase-proximal proteins, is the mechanism for non-lethal activation, opening the methodological development of reversible modification of neuronal function via regulating caspase-proximal proteins.
Authors: Masaya Muramoto, Nozomi Hanawa, Misako Okumura, Takahiro Chihara, Masayuki Miura and Natsuki Shinoda
Journal: eLife 13:RP99650, 2025
DOI: https://doi.org/10.7554/eLife.99650
Press release: 生きる神経細胞の中で区画化された「死の酵素」の活性化
投稿者プロフィール

最新の投稿
 令和7年度(FY2025)2025.06.19Executioner caspase is proximal to Fasciclin 3 which facilitates non-lethal activation in Drosophila olfactory receptor neurons
令和7年度(FY2025)2025.06.19Executioner caspase is proximal to Fasciclin 3 which facilitates non-lethal activation in Drosophila olfactory receptor neurons ノンドメインブログ2023.09.06細胞自動セグメンテーションAI
ノンドメインブログ2023.09.06細胞自動セグメンテーションAI 令和5年度 (FY2023)2023.05.16Caspase cleaves Drosophila BubR1 to modulate spindle assembly checkpoint function and lifespan of the organism
令和5年度 (FY2023)2023.05.16Caspase cleaves Drosophila BubR1 to modulate spindle assembly checkpoint function and lifespan of the organism ノンドメインブログ2022.07.03危険なおもちゃ(カスパーゼ)の扱い方
ノンドメインブログ2022.07.03危険なおもちゃ(カスパーゼ)の扱い方